Location: Home >> Detail
TOTAL VIEWS
Adv Geriatr Med Res. 2026;8(1):20260001. https://doi.org/10.20900/agmr20260001
1 Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Godoy Cruz 2290, Ciudad Autonoma de Buenos Aires C1425FQB, Argentina
2 Systems Biology of Complex Diseases, Centro de Investigacion Translacional en Salud (CENITRES), Universidad Maimónides, Ciudad Autonoma de Buenos Aires C1405BCK, Argentina
* Correspondence: Nicolas Glasbauer
By 2021, twenty-one million people in the US suffered at least one major depressive episode in their lives, while, in 2019, the WHO estimated that 280 million people were living with depression. Recent research may indicate that olfactory dysfunction plays a role in the pathophysiology of depression due to shared neural pathways involving the limbic system. This meta-analysis aims to systematically review the relationship between olfactory dysfunction and depression by analyzing data obtained by objective tests from studies up to January 6, 2024. The overall analysis, including 13 studies relatively homogenous studies without publication bias, showed a slight, nonsignificant decline of olfactory perception in patients with depression vs. controls standardized mean difference (SMD) = −0.137, 95% CI: −0.319 to 0.044; p = 0.137). However, sensitivity analyses using moderators such as gender, age, and type of olfactory test revealed variability in results, with the Sniffin’ Sticks test showing a significant association (SMD = −0.233, 95% CI: −0.454 to −0.012; p = 0.039). These findings suggest that olfactory dysfunction may be associated with depression, particularly when measured with the Sniffin Sticks test. Further research into standardized methodologies is needed to clarify this relationship.
The World Health Organization defines health as “a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity” [1].
The human body is intricately woven, and its proper function depends on the five senses: sight, hearing, touch, taste, and smell. The sense of smell is closely linked to brain regions involved in emotions, learning, memory, and reward, such as the amygdala and orbitofrontal cortex [2-4].
Olfactory dysfunction, characterized by a diminished ability to perceive odors, has been implicated in several neurodegenerative diseases and psychiatric disorders, including depression. Given the shared neural pathways involved in olfaction and emotional processing, specifically the limbic system and prefrontal cortex, it is hypothesized that olfactory dysfunction could play a role in the etiology or exacerbation of depression. [5-7]. Furthermore, recent research suggests that there is a link between olfactory reduction and various neurological and psychological disorders, including depression [2,3,8-10].
Major depressive disorder (MDD) is a prevalent mental health disorder characterized by persistent sadness, loss of interest, and other symptoms that significantly impact individuals’ quality of life. While the exact cause of depression remains unknown, it is likely a complex interplay of genetic, environmental, and neurobiological factors [9,11,12].
Depression affects an estimated 3.8% of the global population, with prevalence rates of 5% among adults (4% in males and 6% in females) and 5.7% among individuals over the age of 60. This prevalence translates to over 290 million people worldwide living with depression [13].
The present meta-analysis aims to systematically review the existing literature to clarify the relationship between depression and olfactory dysfunction, providing an updated quantitative synthesis using a standardized random-effects meta-analytic framework and extending the evidence base beyond prior systematic work [14]. Our study has the main objective of evaluating whether there is a clear relationship between depression and olfactory dysfunction diagnosed by objective tests. In addition, as secondary endpoints, we intend to disclose essential confounders such as age and sex. We contextualize our results with more recent quantitative syntheses with more strict inclusion criteria [15].
A thorough literature search of the online PubMed database covered all papers until January 6, 2024. The search terms were selected to include specific keywords and phrases that encompass a broad range of studies on olfactory perception and its association with depression.
The query was:
(olfactory hedoni* OR “odor perception dysfunction” OR “olfactory dysfunction” OR “smell perception disorder” OR “olfactory perception disorder” OR “anosmia” OR “hyposmia” OR “olfactory impairment” OR “smell impairment” OR “disorder of smell” OR “olfactory sensitivity disorder” OR “olfactory loss” OR “dysosmia” OR “olfactory dysfunctions”) AND (depression OR anxiety) AND human* NOT (mice OR rat* OR Chronic Rhinosinusitis OR tremor OR stomach OR Schizophrenia OR COVID-19 OR Anorexia OR Parkinson OR Alzheimer)
No filters were applied, and from the list of relevant studies, each article was first evaluated by title and abstract. The selected full manuscripts were analyzed in a third step. References from previous systematic reviews were used to corroborate that the search included all the relevant references as recommended [16].
Inclusion and Exclusion CriteriaStudies had to include adolescents and adults (>12 years old). For primary depression, patients should go through a psychological or psychiatric clinical evaluation with the utilization of validated diagnostic tools using the standardized criteria of DSM-IV or DSM-V. Besides, studies included in this analysis had to provide quantitative data about the association between olfactory function and depressive symptoms. Studied must use validated methods of olfactory perception, such as the Sniffin Sticks Test or the University of Pennsylvania Smell Identification Test (UPSIT). Then, we excluded articles if they reported only subjective olfactory information. Studies needed to include a healthy control group, and they were excluded if they used only a self-report to assess patients’ depression. After two reviewers independently screened the titles and abstracts, full–text articles were reviewed to confirm eligibility based on the inclusion criteria. Figure 1 shows a flow chart of the eligibility proces [14,17,18].
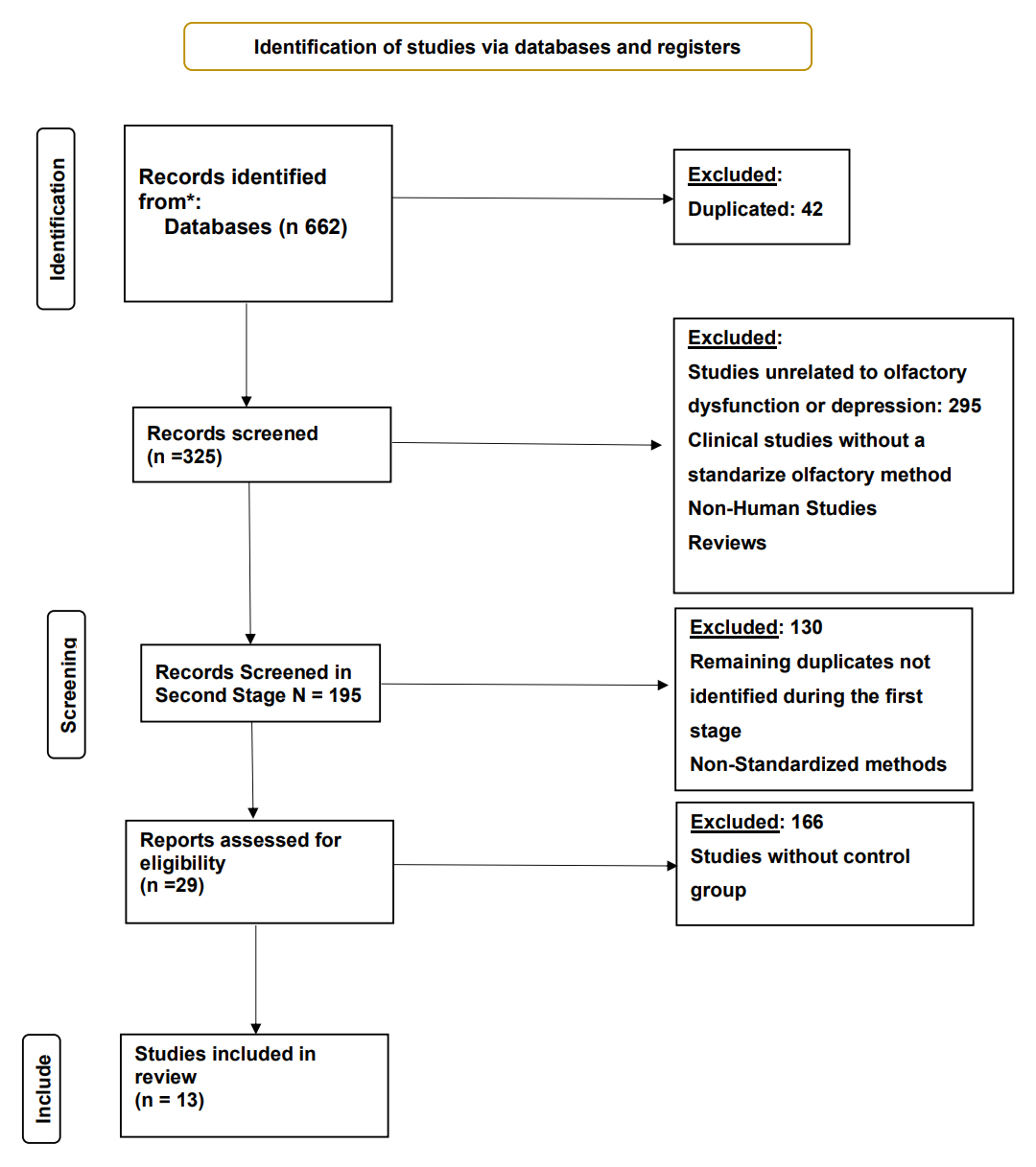 Figure 1. PRISMA flow diagram of study selection. * PUBMED Database. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram summarising identification, screening, eligibility assessment and inclusion of studies. Records were retrieved from PubMed (n = 662). After removal of duplicates (n = 42) and exclusion of records that did not meet the inclusion criteria—e.g., studies unrelated to olfactory dysfunction or depression, non-human studies, reviews, or those using non-standardized olfactory methods—13 studies remained for quantitative synthesis.
Figure 1. PRISMA flow diagram of study selection. * PUBMED Database. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram summarising identification, screening, eligibility assessment and inclusion of studies. Records were retrieved from PubMed (n = 662). After removal of duplicates (n = 42) and exclusion of records that did not meet the inclusion criteria—e.g., studies unrelated to olfactory dysfunction or depression, non-human studies, reviews, or those using non-standardized olfactory methods—13 studies remained for quantitative synthesis.
The analysis was carried out using the SMD as the outcome measure to be independent of the tests used, as the scaling is irrelevant after normalization. A random-effects model was fitted to the data. The amount of heterogeneity was estimated using the DerSimonian-Laird estimator (tau2) [19]. In addition to the estimate of tau2, the Q-test for heterogeneity [20] and the I2 statistic were reported. If any amount of heterogeneity is detected (i.e., tau2 > 0, regardless of the results of the Q-test), a prediction interval for the true outcomes was also provided.
Studentized residuals and Cook’s distances are used to examine whether studies may be outliers and/or influential in the context of the model. Studies with a studentized residual larger than the 100 × (1 − 0.05/(2 × k))th percentile of a standard normal distribution are considered potential outliers (i.e., using a Bonferroni correction with two-sided alpha = 0.05 for k studies included in the meta-analysis). Studies with a Cook’s distance more extensive than the median plus six times the interquartile range of the Cook’s distances are considered influential. Besides, the analysis was repeated, removing one study at a time to check for homogeneity of the summarized data. Begg-Mazundar (BM) rank correlation test and Egger’s regression test, using the standard error of the observed outcomes as a predictor, are used to check for funnel plot asymmetry [19,21].
Extracted data included demographic characteristics (mean age, gender distribution), sample sizes for case and control groups, and summary statistics (means and standard deviations) for olfactory measures, including the particular test employed according to depression status (Table 1).
Calculations were performed using both free JAMOVI v2.4.11 package available at https://www.jamovi.org 31/01/2024 and OpenMeta(Analyst) available at http://www.cebm.brown.edu/openmeta/# 15/04/2025 .
A total of 13 studies representing 478 471 cases and 498 control subjects were included in this meta-analysis. We calculated each study’s SMD between both groups, Controls or patients with depression, and a random–effect model was applied to account for potential variability among studies. We obtained an estimated average SMD of −0.137 (95% CI: −0.319 to 0.044), suggesting a slight negative but non-significant-association between olfactory dysfunction and depression (Z = −1.49, p = 0.137) (Figure 2).
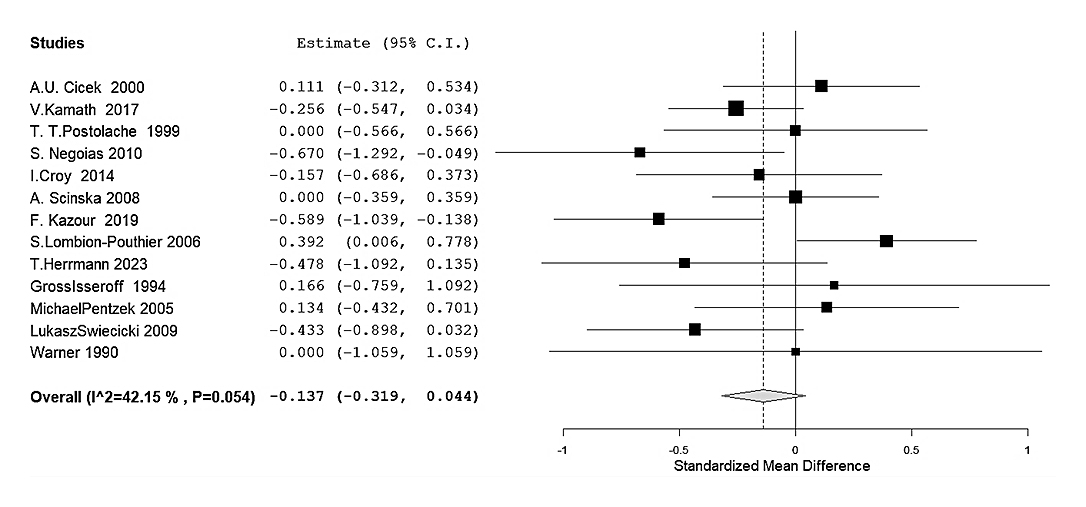 Figure 2. Pooled association between olfactory function and depression (all objective tests combined). Random-effects forest plot of the SMD in overall olfactory performance between adults and adolescents with depression and health controls across 13 studies. Negative SMD values indicate poorer olfactory function in the depression group. Squares represent study-specific SMDs (size proportional to inverse-variance weight), and horizontal lines show 95% confidence intervals (CI). The diamond depicts the pooled estimate (SMD = −0.137, 95% CI −0.319 to 0.044, p = 0.137). The dashed vertical line marks no effect (SMD = 0). Moderate heterogeneity was present (Q = 20.9, p = 0.054; I2 = 42%).
Figure 2. Pooled association between olfactory function and depression (all objective tests combined). Random-effects forest plot of the SMD in overall olfactory performance between adults and adolescents with depression and health controls across 13 studies. Negative SMD values indicate poorer olfactory function in the depression group. Squares represent study-specific SMDs (size proportional to inverse-variance weight), and horizontal lines show 95% confidence intervals (CI). The diamond depicts the pooled estimate (SMD = −0.137, 95% CI −0.319 to 0.044, p = 0.137). The dashed vertical line marks no effect (SMD = 0). Moderate heterogeneity was present (Q = 20.9, p = 0.054; I2 = 42%).
The remotion of one study at a time supports this general conclusion, indicating that no study heavily modified the pooled estimate except one [22] with an opposite effect direction (Figure 3). There is no indication of publication bias either in statistical tests (BM Rank Correlation: −0.103, p: 0.675, Egger’s Regression: −0.137, p: 0.891) or funnel plot (Figure 4) [23,24].
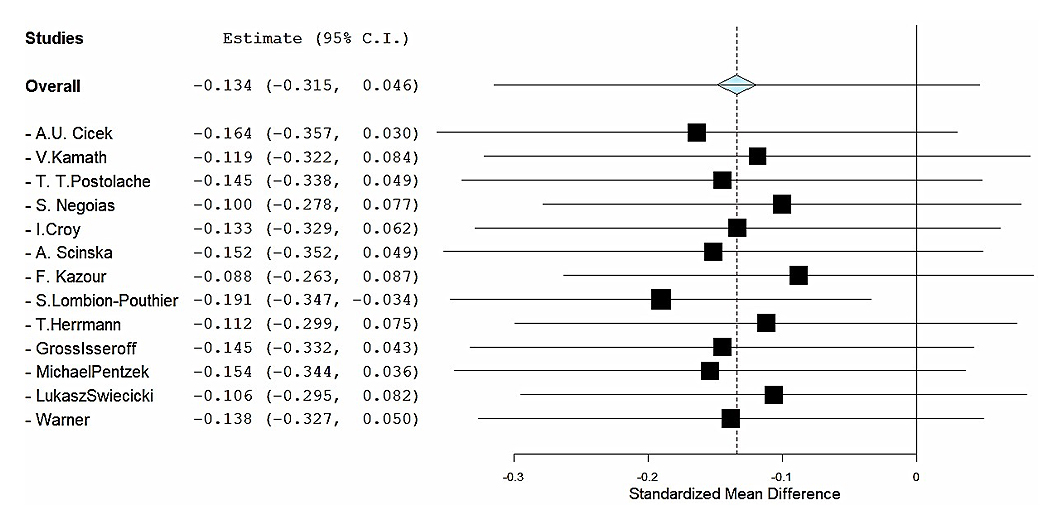 Figure 3. Leave-one-out influence analysis. Forest plot showing the pooled SMD in olfactory performance between depressed patients and controls after sequentially omitting each study. For every iteration the meta-analysis is re-run without the indicated study, generating a new pooled SMD (black square) with its 95% confidence interval (horizontal line). The grey diamond at the top represents the original overall estimate (all 13 studies included: SMD = −0.137, 95% CI −0.319 to 0.044). Negative SMD values indicate poorer olfactory function in the depression group.
Figure 3. Leave-one-out influence analysis. Forest plot showing the pooled SMD in olfactory performance between depressed patients and controls after sequentially omitting each study. For every iteration the meta-analysis is re-run without the indicated study, generating a new pooled SMD (black square) with its 95% confidence interval (horizontal line). The grey diamond at the top represents the original overall estimate (all 13 studies included: SMD = −0.137, 95% CI −0.319 to 0.044). Negative SMD values indicate poorer olfactory function in the depression group.
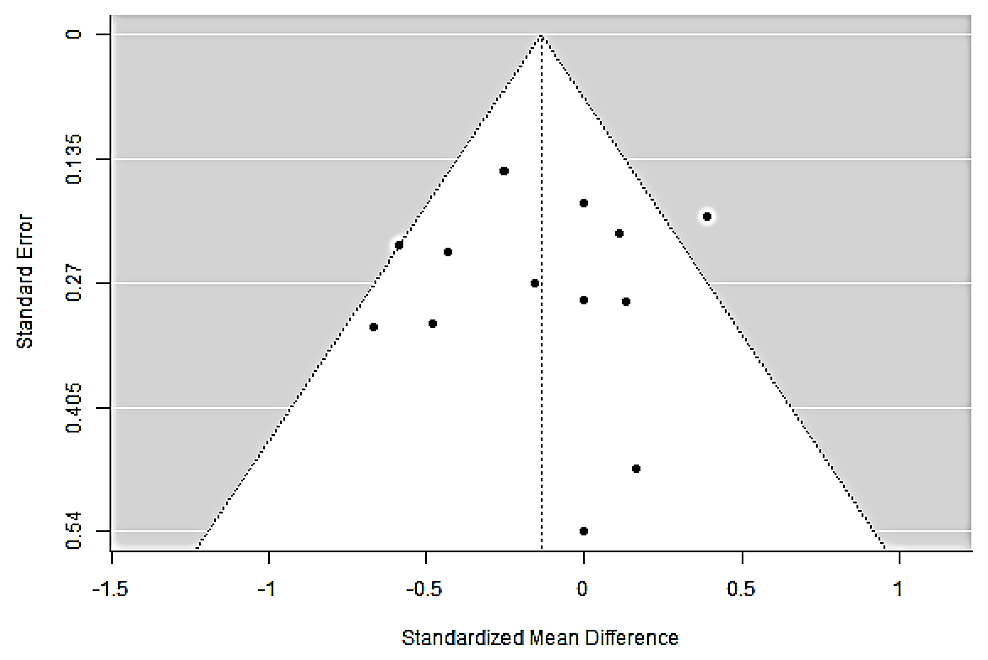 Figure 4. Funnel plot for assessment of small-study effects/publication bias. Scatterplot of the 13 study-level SMDs (x-axis) against their standard errors (y-axis, inverted). The white triangle indicates the region within which 95% of study estimates are expected to fall in the absence of publication bias and heterogeneity. Dotted vertical line marks the pooled effect (SMD ≈ −0.14). The symmetric distribution of points, with studies appearing on both sides of the pooled estimate at all precision levels, suggests no overt small-study effects. This visual impression is supported by formal tests (Begg–Mazumdar rank correlation τ = −0.103, p = 0.675; Egger regression intercept = −0.137, p = 0.891; two-sided). Abbreviations: SMD, standardized mean difference.
Figure 4. Funnel plot for assessment of small-study effects/publication bias. Scatterplot of the 13 study-level SMDs (x-axis) against their standard errors (y-axis, inverted). The white triangle indicates the region within which 95% of study estimates are expected to fall in the absence of publication bias and heterogeneity. Dotted vertical line marks the pooled effect (SMD ≈ −0.14). The symmetric distribution of points, with studies appearing on both sides of the pooled estimate at all precision levels, suggests no overt small-study effects. This visual impression is supported by formal tests (Begg–Mazumdar rank correlation τ = −0.103, p = 0.675; Egger regression intercept = −0.137, p = 0.891; two-sided). Abbreviations: SMD, standardized mean difference.
Heterogeneity was moderate, with an I2 of 42.15% (τ2\tau2τ2 = 0.0435, p = 0.054), indicating some variability across studies that may be attributable to random sampling error. However, different moderators were applied in sensitivity studies to ensure that all variables potentially affecting the link between olfactory dysfunction and depression were considered.
Using gender distribution (percentage of females over males) as a continuous moderator, the pooled SMD reached an estimate of −0.120 (SE = 0.174, Z = −0.687, p = 0.492) and a nonsignificant moderator effect (−0.060, SE = 0.469, p = 0.898). This result suggests that the percentage of sexual diversity of participants in each study did not statistically modify the relationship between olfactory dysfunction and depression, and there was no sexual dimorphism in the association [25] (Figure 5).
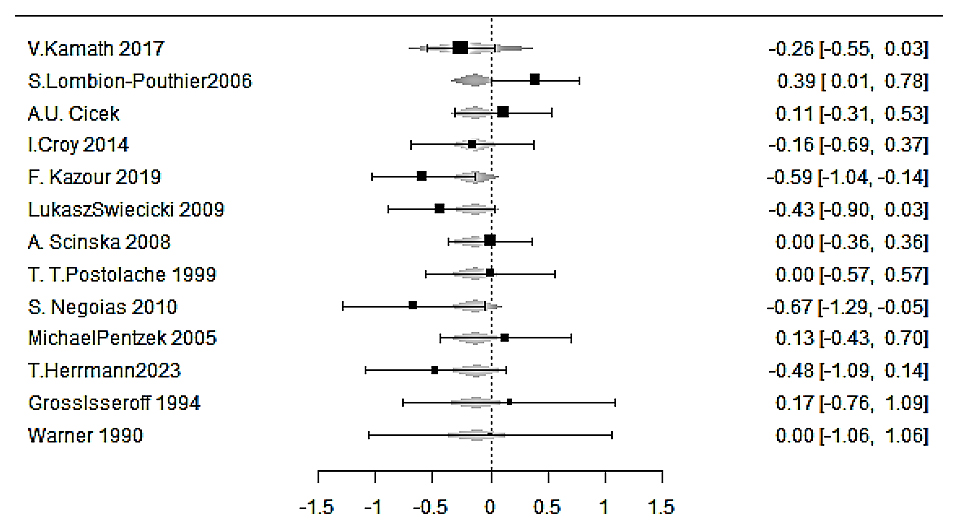 Figure 5. Forest plot from the meta-regression testing the influence of participant sex on the olfactory–depression association. Study-level SMDs in olfactory performance (depression vs. control) are displayed after fitting a random-effects meta-regression in which each study is weighted by inverse variance, and the proportion of female participants is entered as a continuous moderator. Squares mark the adjusted SMD for each study; horizontal bars show 95% CI. Negative SMD values indicate poorer olfactory function in the depression group. The overall moderator was not significant (slope = −0.060 ± 0.469, p = 0.898), and the pooled effect at the mean sex ratio remained nonsignificant (estimate = −0.120 ± 0.174, Z = −0.69, p = 0.492), indicating no evidence of sexual dimorphism in the olfactory deficit associated with depression. Residual heterogeneity was low (τ2 = 0.034; I2 ≈ 36%).
Figure 5. Forest plot from the meta-regression testing the influence of participant sex on the olfactory–depression association. Study-level SMDs in olfactory performance (depression vs. control) are displayed after fitting a random-effects meta-regression in which each study is weighted by inverse variance, and the proportion of female participants is entered as a continuous moderator. Squares mark the adjusted SMD for each study; horizontal bars show 95% CI. Negative SMD values indicate poorer olfactory function in the depression group. The overall moderator was not significant (slope = −0.060 ± 0.469, p = 0.898), and the pooled effect at the mean sex ratio remained nonsignificant (estimate = −0.120 ± 0.174, Z = −0.69, p = 0.492), indicating no evidence of sexual dimorphism in the olfactory deficit associated with depression. Residual heterogeneity was low (τ2 = 0.034; I2 ≈ 36%).
When analyzing the mean age of participants as a continuous moderator, the estimate was −0.280 (SE = 0.296, Z = −0.947, p = 0.343) and a nonsignificant effect for the age moderator was found (estimate = 0.0033, SE = 0.0064, p = 0.611).
In searching for the source of heterogeneity, we then evaluated the type of olfactory test as a factor to determine whether the assessment method influenced the observed association between olfactory dysfunction and depression. This analysis aimed to identify if specific olfactory tests, such as the UPSIT or the Sniffin’ Sticks test, yielded different results, potentially impacting the reliability and interpretation of the association.
On the one hand, eight studies utilizing the sniffing stick test were included in the analysis.
The random-effects model provided an average SMD of −0.233 (95% CI: −0.454 to −0.012), indicating a statistically significant negative association between olfactory function and depression (Z = −2.07, p = 0.039). The Q-test for heterogeneity was not significant, although the I2 statistic of 38.72% may indicate a low/moderate heterogeneity. Although some variability among studies may exist, discarding the overall trend observed in the association is not justified. Overall, these results suggest that patients suffering from depression exhibited a meaningful reduction in olfactory function compared to controls when measured using the Sniffin Sticks test. (Figure 6). On the other hand, four studies using the UPSIT were analyzed, yielding an average SMD of −0.167 (95% CI: −0.409 to 0.075). This result was not statistically significant (Z = −1.35, p = 0.176), indicating that the UPSIT might not detect a meaningful association between olfactory dysfunction and depression. The analysis showed no significant heterogeneity (Q3 = 1.293, p = 0.731) and an I2 of 0%, suggesting homogeneity across studies. This result may indicate that the results were consistent, and the UPSIT test might be less sensitive (Figure 7).
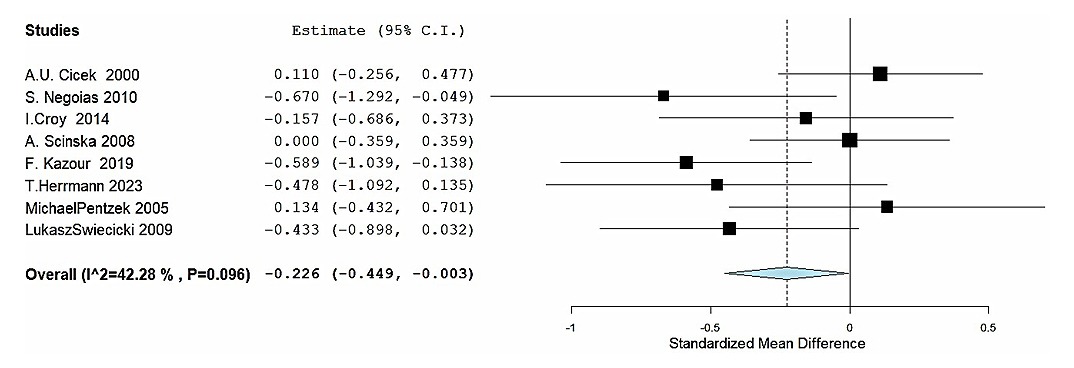 Figure 6. Sub-group analysis of Sniffin’ Sticks® studies. Forest plot restricted to the eight studies that assessed olfactory function with the Sniffin’ Sticks® test. Negative SMD values indicate poorer olfactory function in the depression group. The pooled random-effects estimate shows a significant association (SMD = −0.233, 95% CI −0.454 to −0.012; p = 0.039). Low-to-moderate heterogeneity was detected (I2 = 39%).
Figure 6. Sub-group analysis of Sniffin’ Sticks® studies. Forest plot restricted to the eight studies that assessed olfactory function with the Sniffin’ Sticks® test. Negative SMD values indicate poorer olfactory function in the depression group. The pooled random-effects estimate shows a significant association (SMD = −0.233, 95% CI −0.454 to −0.012; p = 0.039). Low-to-moderate heterogeneity was detected (I2 = 39%).
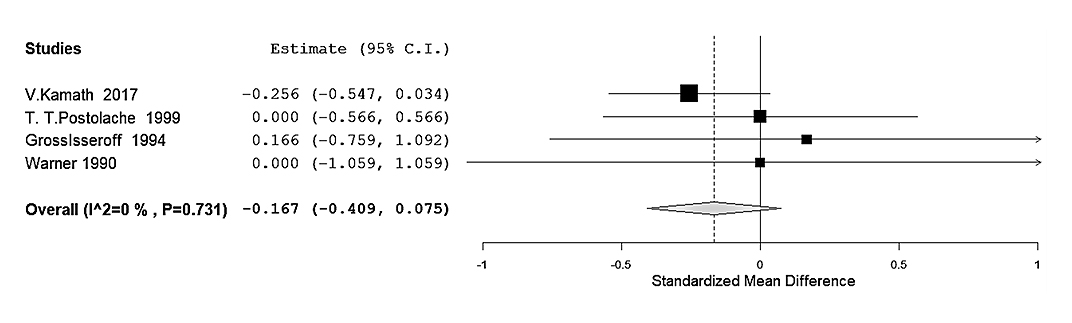 Figure 7. Sub-group analysis of UPSIT studies. Forest plot restricted to the four studies that employed the UPSIT. Negative SMD values indicate poorer olfactory function in the depression group. The pooled estimate did not reach statistical significance (SMD = −0.167, 95% CI −0.409 to 0.075; p = 0.176). No appreciable heterogeneity was observed (Q = 1.29, p = 0.731; I2 = 0%).
Figure 7. Sub-group analysis of UPSIT studies. Forest plot restricted to the four studies that employed the UPSIT. Negative SMD values indicate poorer olfactory function in the depression group. The pooled estimate did not reach statistical significance (SMD = −0.167, 95% CI −0.409 to 0.075; p = 0.176). No appreciable heterogeneity was observed (Q = 1.29, p = 0.731; I2 = 0%).
This meta-analysis of relatively homogenous studies reveals that the general association between olfactory dysfunction and depression may not reach overall statistical significance. However, results may depend on the test used. A significant decrease in olfactory perception of patients with depression was observed in the pooled eight studies using the Sniffin’ Sticks test. In contrast, no difference was observed in studies using UPSIT, indicating that the Sniffin’ Sticks may present different results than UPSIT because it provides a multidimensional assessment of olfaction (Threshold, discrimination, identification), whereas UPSIT primarily reflects odor identification threshold. A note of caution should be added, however, as only four studies using UPSIT with 120 controls and 169 patients with depression were included, and a lack of statistical power may explain the results. Interestingly, sexual dimorphism and age do not explain the differences in odor perception between subjects’ groups [14,25].
Nevertheless, the significant findings pointed out by the Sniffin’ Sticks test studies are supported by some scientific findings that aim to find a possible relationship between odor perception deficit and depression [5,10,26].
On the one hand, the limbic system’s involvement in olfaction and emotional regulation supports the hypothesis that olfactory dysfunction may contribute to depressive symptoms [10]. The limbic system, including the amygdala, hippocampus, and orbitofrontal cortex, plays a key role in olfactory processing and mood regulation [5,10]. Therefore, dysfunction in these regions may lead to depressive symptoms and impaired olfactory function. The elevation of inflammatory cytokines, stress–related hormonal changes, and neuroinflammation such as interlukin-6 (IL6) and tumor necrosis factor-alpha (TNFa) may contribute to olfactory and mood dysfunction [10,26].
On the other hand, [27] reported no significant relationship, which may highlight the potential influence of age on an olfactory deficit contradicting the lack of relationship of age-related changes in our meta-analysis.
Patients with acute major depression had reduced olfactory bulb volume, suggesting that neuroanatomical and structural olfactory pathways changes may underlie olfactory dysfunction in depression [2].
It was reported that patients with MDD exhibited changes in olfactory sensitivity after they finished antidepressant treatment, this suggests a relationship between state-dependent depression and olfactory function [28].
Distinct mechanisms of olfactory dysfunction across different psychiatric conditions were implied by others [29]. On the premise that the olfactory identification deficit was more pronounced in patients with bipolar disorder compared to patients with major depression or anxiety disorders.
Furthermore, research on the cognitive components of smell in depression indicates that deficits in odor discrimination and identification may reflect more general attentional and processing-speed impairments frequently observed in MDD. Cognitive variables must be considered, as they may affect the interpretation of olfactory test findings.
The results obtained with more sensitive tests, such as sniffing sticks, suggest that people with depression might experience a significant reduction in olfactory function.
Several limitations were encountered during the meta-analysis; although heterogeneity was moderate, it suggests variability in study design, population characteristics, and assessment methods. In addition, the limited number of studies using UPSIT may limit the interpretability of results for this test.
To conclude, this meta-analysis provides evidence of a test—dependent association between depression and olfactory dysfunction, with more consistent differences observed in studies using multidimensional objective assessment. Overall, our findings support the hypothesis that olfactory deficits may be a clinical characteristic associated with depression. However, olfactory impairment is non–specific and cannot be considered the only marker for MDD based on the predominantly cross–sectional evidence. Clinical utility for early detection, prevention, or treatment selection remains uncertain and should be addressed by longitudinal and interventional studies before recommending routine implementation. Future research with larger sample sizes and objective and standardized smell perception testing methods is needed to further explore its association with neurological conditions and underlying mechanisms.
Including olfactory testing in daily practice may present new approaches to improving people’s lives, preventing depressive episodes, and developing new treatment strategies.
This study is a meta-analysis of previously published research. All individual studies included in the analysis reported approval by their respective institutional review boards or ethics committees, and informed consent was obtained from participants as described in the original publications. As the present work involved secondary synthesis of published, de-identified data, no additional ethical approval was required.
Declaration of Helsinki STROBE Reporting GuidelineThis study adhered to the Helsinki Declaration. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) reporting guideline was followed.
All data generated from the study are available in the manuscript or supplementary files.
Conceptualization, NDG and CJP; Methodology, NDG and CJP; Validation, NDG and CJP; Formal Analysis, NDG and CJP; Investigation, NDG and CJP; Data Curation, NDG and CJP; Writing—Original Draft Preparation, NDG and CJP; Writing—Review & Editing, NDG and CJP; Visualization, NDG and CJP; Supervision, CJP; Project Administration, CJP.
CJP: Carlos J. Pirola
NDG: Nicolas D. Glasbauer
NG has a fellowship of National Scientific and Technical Research Council (CONICET) in association with Maimonides University. CJP belongs to CONICET. They declare no conflicts of interest.
Intramural grants from Maimonides University partially supported this work.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
Glasbauer N, Pirola CJ. Olfaction under the shadow of depression: Objective evidence from a comprehensive meta-analysis. Adv Geriatr Med Res. 2026;8(1):e260001. https://doi.org/10.20900/agmr20260001.

Copyright © Hapres Co., Ltd. Privacy Policy | Terms and Conditions