Location: Home >> Detail
TOTAL VIEWS
Adv Geriatr Med Res. 2025;7(2):e250009. https://doi.org/10.20900/agmr20250009
1 School of Health and Exercise Sciences, University of British Columbia Okanagan, Kelowna, BC V1V 1V7, Canada
2 Interdisciplinary School of Health, University of Quebec in Outaouais, Gatineau, QC J8X 3X7, Canada
3 Faculty of Physical Activity Sciences, University of Sherbrooke, Sherbrooke, QC J1K 2R1, Canada
* Correspondence: Jonathan P. Little.
Type 2 diabetes (T2D) remission, defined as an A1c level below 6.5% without the use of glucose-lowering medications for at least three months, is now recognized as a viable management strategy for the condition. Despite the growing popularity of intermittent fasting (IF), limited studies investigate whether IF can induce T2D remission, especially in real-world settings.
Case presentation: This case study documents a 47-year-old female living with T2D for the past 15 years who achieved T2D remission using IF. In February 2024, the patient was using several glucose-lowering drugs before starting an IF protocol. The protocol involved 13–83 h fasting, 2–3 times/week beginning on March 14th, 2024, with simultaneous discontinuation of medication usage and the use of continuous glucose monitoring (CGM) for guidance. Before starting IF, her 24-h average CGM glucose was 12.7 mmol/L, with 18% time in range and an eA1c of 9.6%. After three months of IF, she lost a total of 13.8 kg and achieved a CGM-derived average glucose of 6.9 mmol/L, spending 98% time in range with an eA1c of 5.97%. Smartwatch data also indicated that the patient maintained an active lifestyle, averaging 280 ± 33 min/week of physical activity. Finally, the patient’s resting heart rate improved after achieving remission (71 ± 2 bpm to 59 ± 3 bpm).
Conclusion: This case study underlines the potential of achieving T2D remission with IF and physical activity in real-world settings outside clinical and research environments. It also highlights the utility of using CGM to facilitate self-management.
Type 2 diabetes (T2D)—characterized by impaired glycemic control—continues to be classified as a “chronic and progressive condition” [1,2], often necessitating increased medication use over time. Increased medication usage increases polypharmacy risk, which is a known risk factor for hospitalization and premature mortality [3,4]. Finding non-pharmaceutical strategies to manage T2D to reduce the risk of polypharmacy and its associated costs is crucial.
In their first clinical practice guidelines on T2D remission, Diabetes Canada defined remission as the achieving an A1c below 6.5% without glucose-lowering medication use for 3 consecutive months [5]. T2D remission can be achieved using bariatric/metabolic surgery [5], hypocaloric meal-replacement diets [6,7], low-carbohydrate diets [8,9], and lifestyle interventions combining physical activity and nutrition [10,11]. Weight loss (~15 kg) appears to be a key contributor to achieving T2D remission, highlighting the potential for alternative nutrition therapies such as intermittent fasting (IF) for achieving remission [12,13]. Although no RCT has investigated the potential of IF for achieving T2D remission, Yang et al. (2023) demonstrated that 47.2% of individuals following a 3-month intermittent low-calorie intake regimen [i.e., six cycles of 5 days of calorie-restricted diet (∼840 kcal/day) followed by 10 days ad libitum feeding] achieved T2D remission [14]. Several case reports/series also suggest that combining IF with a low-carbohydrate diet could help manage glycemic control and reduce glucose-lowering medication use [15–17]. However, these case reports stem from clinical settings using supervised multidisciplinary approaches (e.g., nutritional seminars, regular meetings with healthcare professionals). As such, it remains unknown if IF can be effective for T2D remission in real-world settings. Given the popularity of IF and the potential for individuals living with T2D to self-manage their condition [9], exploring the real-world utility of IF approaches for T2D remission could help inform treatment options. Furthermore, no existing study has utilized continuous glucose monitoring (CGM) to assess key aspects of glucose regulation (e.g., glycemic variability, time in range, risk of hypoglycemia) in the context of IF and T2D remission. The present case study describes the feasibility of achieving T2D remission in real-world settings using IF, while emphasizing a potentially important role of CGM in helping patients safely achieve their diabetes self-management goals.
This case study documents the story of a 47-year-old female living with T2D for the past 15 years (i.e., diagnosed in 2009). From 2004 to 2017, the patient managed her T2D primarily through exercise (i.e., 30–45 min, 2–3 times/week). However, after gaining over 40 pounds in 2017, her glucose levels were suboptimal, leading to the introduction of Metformin (500 mg DIE), which was later increased to 850 mg twice daily. In January 2020, self-guided use of CGM showed an average blood glucose of 9.2 mmol/L. From 2020 to 2023, she was prescribed Empagliflozin (10 mg DIE), which was discontinued and replaced with Gliclazide (30 mg DIE) and Ozempic. Ozempic was stopped after 6 months due to side effects (nausea, vomiting). In December 2023, despite taking Metformin (850 mg BID) and Gliclazide MR (60 mg BID), her average glucose was 11.9 mmol/L. In February 2024, the patient was prescribed 10 units of degludec insulin (every other day). She therefore decided to take the matter into her own hands and started researching more aggressive dietary approaches online (see below).
Past and Current NutritionSince her diagnosis, the patient described eating a mixed diet largely based on Canada’s Food Guide, consuming a healthy protein source for lunch and dinner (e.g., chicken, salmon, ground beef) combined various vegetables (e.g., broccoli, spinach, carrots, eggplant, etc.) and starches (e.g., potatoes, rice, pasta, etc.). She also reported ordering fast food (e.g., pizza, burgers) and sugary drinks (hot beverages) as well as consuming processed foods (e.g., chips) regularly. This represents the food consumed during Phase 1 (see timeline Figure 1).
March 4th, 2024 the patient decided to take action on their own and started integrating diet tips seen on the internet. Specifically, she started having savoury breakfasts containing higher fat, protein and fiber, increasing her daily consumption of veggies, consuming 1 tablespoon of vinegar every day, and consuming food in a specific order (i.e., fiber, protein, fat, starchy carbs followed by sugar—if any; Figure 1-Phase 2). She used these strategies until March 13th, after which she started IF to further improve her “glucose spikes”. On March 14th, she started with time-restricted eating by skipping breakfast and also stopped taking all her medication. She gradually increased her fasting period until she performed her first 24 h fast on March 18th, followed by 42 h fast from March 20th to 22nd 2024. She continued various continuous fasts ranging from 13 h to 83 h with a subsequent eating window of ~8–10 h (full fasting timeline is available in Supplementary Material Figure S1). Fasting periods—including both intermittent and short-term fasting as defined by Kloppod et al., 2024 [18]—were adapted based on the participant’s subjective experiences, including energy levels, daily obligations, and work schedule, highlighting the real-world applicability of this case study. On average, she fasted a weekly total of 125 ± 13 h (min-max: 98 h and 22 min to 140 h and 44 min) representing 74% of the time. During the 8–10 h feeding windows, she consumed a low-carbohydrate high-fat (LCHF) diet consisting of two meals (5 h apart) collectively containing <30 g of carbohydrates, focusing on fiber (vegetables above ground, cruciferous, sauerkraut), healthy fats (e.g., avocado, olives, olive oil, butter, Brazil nuts, macadamia nuts, pistachios), and proteins (chicken, salmon, eggs). During her fasts, she would consume black coffee, pink Himalayan salt, and occasionally pickle juice. (See timeline Figure 1-Phase 3). To keep track of her fasting period, the patient used the LIFE Fasting Timer & Tracker. The patient used a Freestyle Libre 2 CGM (Abbott Diabetes Care Inc., Alameda, CA, USA; details below) and regularly monitored their glucose levels using the FreeStyle Libre 2 app.
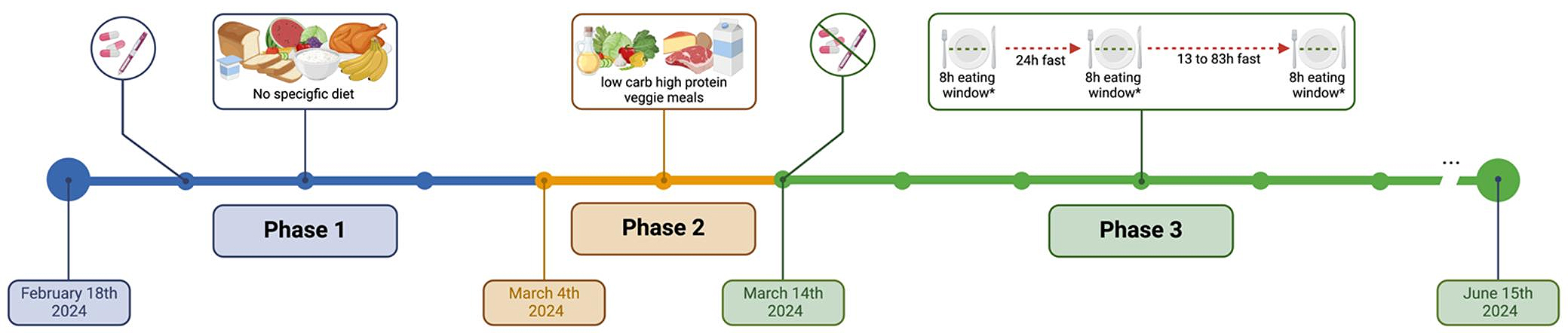 Figure 1. Timeline of her dietary change. Phase 1 = No specific diet or dietary pattern was used during this phase. During Phase 1 the patient was taking; Metformin (850 mg BID), Gliclazide MR (60 mg BID), and 10 units of degludec insulin every other day); Phase 2 = The patient followed various tips and tricks seen online, such as savoury breakfast with high fiber and low carbohydrates, etc.; Phase 3 = The patient started with a 24 h fast following by 13 h to 83 h fast separated by an ~8 h eating window. * During the 8 h eating window the participant aimed to eat a low-carbohydrate high-fat diet. At the beginning of phase 3, the patient discontinued all of their glucose-lowering medications. Created with BioRender.com
Figure 1. Timeline of her dietary change. Phase 1 = No specific diet or dietary pattern was used during this phase. During Phase 1 the patient was taking; Metformin (850 mg BID), Gliclazide MR (60 mg BID), and 10 units of degludec insulin every other day); Phase 2 = The patient followed various tips and tricks seen online, such as savoury breakfast with high fiber and low carbohydrates, etc.; Phase 3 = The patient started with a 24 h fast following by 13 h to 83 h fast separated by an ~8 h eating window. * During the 8 h eating window the participant aimed to eat a low-carbohydrate high-fat diet. At the beginning of phase 3, the patient discontinued all of their glucose-lowering medications. Created with BioRender.com
Body composition (e.g., lean body mass and fat mass) was estimated using Dual Energy X-ray absorptiometry (iDXA; GE Healthcare, Chicago, IL, USA; EnCORE Version 16 software; GE Healthcare, Madison, WI, USA). The patient had data from January 20th, 2020, April 11th, 2024, and June 18th, 2024. The participant’s weight remained relatively stable from 2020 to the beginning of 2024 (i.e., fluctuation ≤ 1 kg) before implementing IF, which allowed the assessment of the body composition change before and during two-time points (i.e., 28 and 97 days after the beginning of the fast).
Resting heart rate (HR) and heart rate variability (HRV) before and after the beginning of the nutritional change were also recorded on their Apple Watch (Serie 7, Cupertino, CA, USA). Specifically, the average resting HR for each day and averages for phase 1, phase 2, and phase 3 were extracted.
Data from CGM were obtained using the Freestyle Libre 2 from February 18th, 2024, to June 15th, 2024. The raw data from the Freestyle Libre 2 were analyzed using the Diametric platform (University of Exeter) and presented in accordance with consensus guidelines for analyzing and interpreting CGM data [19]. Furthermore, as suggested by Riddle and colleagues (2022) in a consensus report on T2D-remission, estimated A1c (eA1C; also referred to as glucose management indicator [GMI]), was used as a surrogate of traditional A1c [20].
The patient was a 47-year-old female diagnosed with T2D for the past 15 years with a BMI of 34.4 kg/m2 and an eA1c of 9.6% (Table 1). More information can be found in the case presentation section.
Change in Body CompositionBefore starting IF, the patient weighed 100.6 kg, with an estimated 60.9 kg of lean mass and 36.1 kg of fat mass. After 28 days of IF and LCHF diet, the patient lost 9.7 kg with 5.8 kg of total fat mass and 2.8 kg of lean body mass but without notable changes in visceral adipose tissue (-32 g). After 2 more months (69 days) the patient lost another 4.1 kg (13.8 kg total in 97 days) while preserving her lean body mass (+0.14 kg) and lost almost 1 pound of (-442 g) of visceral adipose tissue (Figure 2).
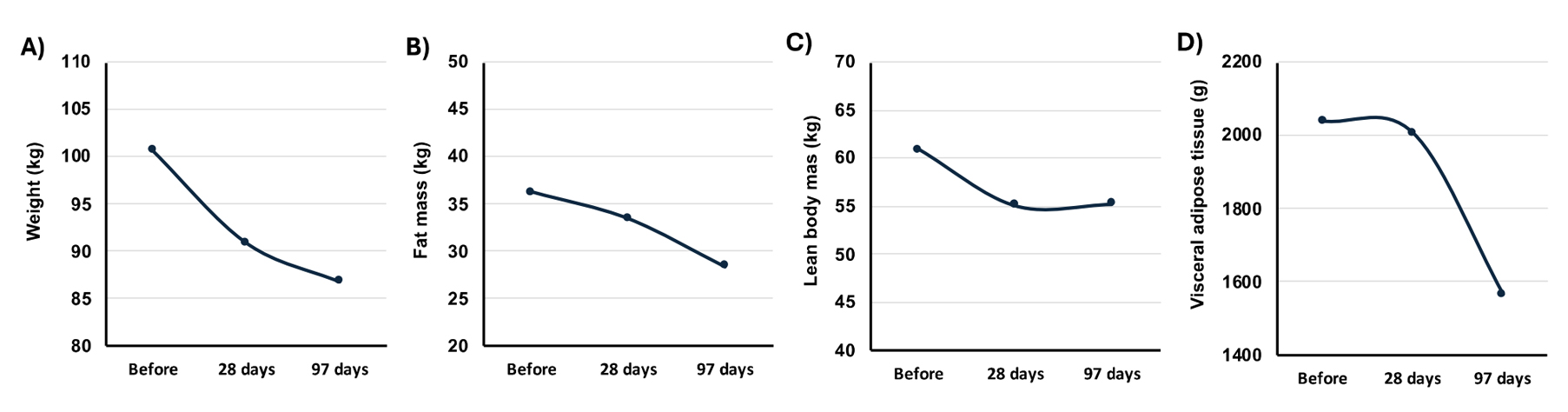 Figure 2. Change in body composition after 28 and 97 days of intermittent fasting. (A) Change in body weight at baseline, Day 28, and Day 93; (B) Change in fat mass at baseline, Day 28, and Day 93; (C) Change in lean body mass at baseline; Day 28, and Day 93; (D) Change in visceral adipose tissue at baseline, Day 28, and Day 93. Before/baseline = January 7th, 2020 (It is to be noted that the weight of the participant didn’t change from 2020 to the beginning of 2024 before implementing various dietary approaches (i.e., fluctuation ≤ 1 kg); 28 days = April 11th; 97 days = June 18th, 2024.
Figure 2. Change in body composition after 28 and 97 days of intermittent fasting. (A) Change in body weight at baseline, Day 28, and Day 93; (B) Change in fat mass at baseline, Day 28, and Day 93; (C) Change in lean body mass at baseline; Day 28, and Day 93; (D) Change in visceral adipose tissue at baseline, Day 28, and Day 93. Before/baseline = January 7th, 2020 (It is to be noted that the weight of the participant didn’t change from 2020 to the beginning of 2024 before implementing various dietary approaches (i.e., fluctuation ≤ 1 kg); 28 days = April 11th; 97 days = June 18th, 2024.
In phase 1, from February 18th to March 3rd, 2024, 24-h average glucose was 12.7 mmol/L, with a SD of 2.7, and an eA1c of 9.60% (Table 2; phase 1–15 days). During that time, despite being on Metformin (850 mg BID), Gliclazide MR (60 mg BID), and 10 units of insulin (every other day) she spent >80% of her time in hyperglycemia and >30% in severe hyperglycemia (>13.9 mmol/L). In phase 2, from March 4th to March 14th, 2024 (10 days), her 24 h average glucose decreased to 8.43 mmol/L with only 16% of time in hyperglycemia. During phase 3, from March 14th to June 15th, 2024 (94 days), she had an average glucose of 6.9 mmol/L, an SD of 1.3 with an eA1c of 5.97%, despite total discontinuation of her medication (Table 2). During this period, the patient spent 98% of her time in range with 0.4% below range and 1.6% above range. No hypoglycemic events or symptoms were noted. A visual representation of the CGM curve from these three phases is presented in Figure 3. On June 11th, 2024, 89 days after discontinuing all her glucose-lowering medication, the patient’s lab-based A1c was 5.8%, confirming T2D remission. During her longest fast (May 19th to May 23rd, 2024; 83 h), the patient’s average glucose was 5.3 mmol/L with the lowest glucose value at 4.1 mmol/L, SD of 0.7 and 100% of her time spent in range (See Supplementary Material Figure S2).
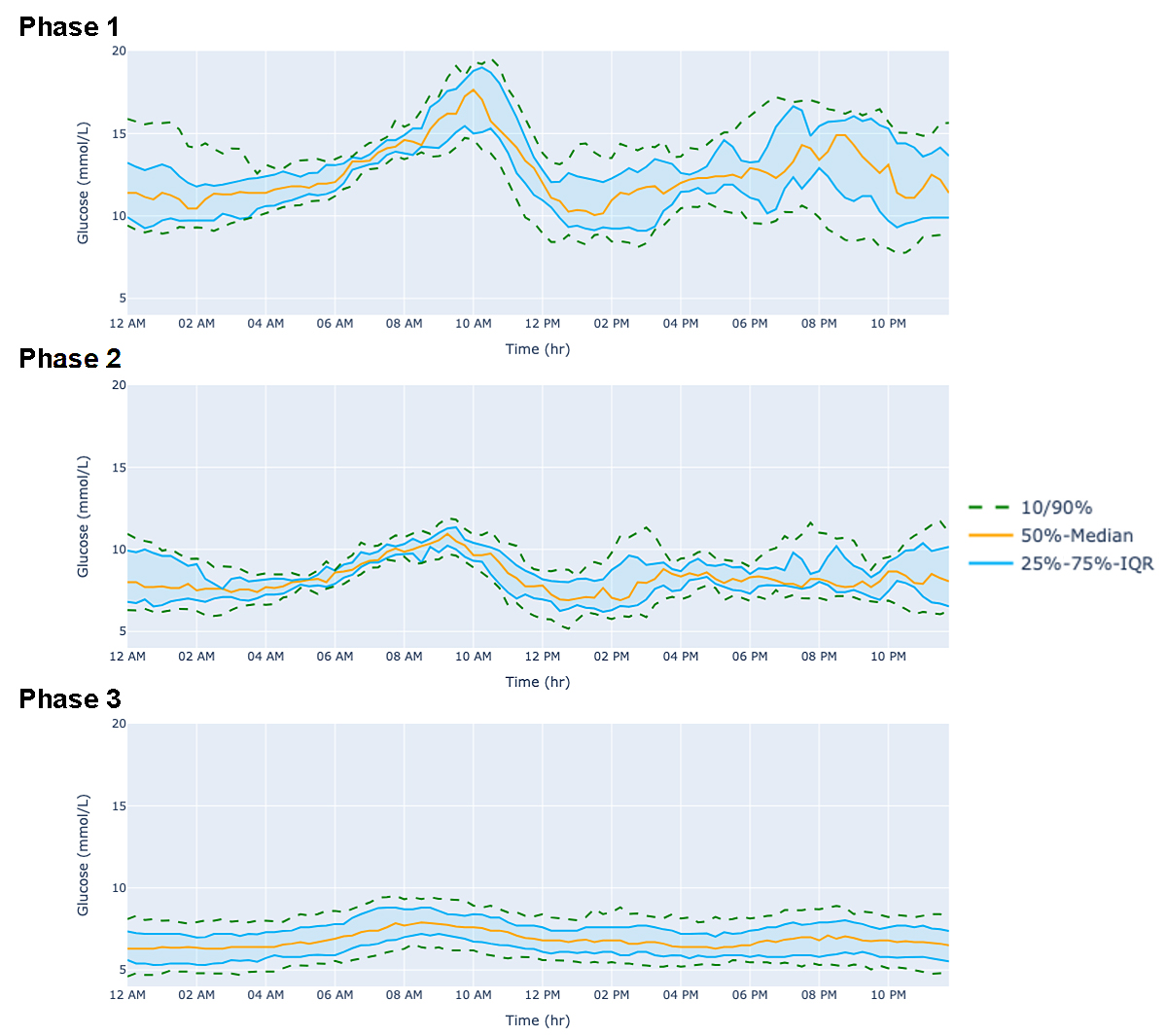 Figure 3. Change in 24-h CGM-derived glucose curve with diet and intermittent fasting. Data are presented as median/25%–75% IQR/10%–90%. (Phase 1) No specific diet from February 18th to March 3rd, 2024; (Phase 2) Following various eating tips from March 4th to March 14th; (Phase 3) Progressive intermittent fasting from 12 h fast to 83 h fast from March 15th to June 15th, 2024.
Figure 3. Change in 24-h CGM-derived glucose curve with diet and intermittent fasting. Data are presented as median/25%–75% IQR/10%–90%. (Phase 1) No specific diet from February 18th to March 3rd, 2024; (Phase 2) Following various eating tips from March 4th to March 14th; (Phase 3) Progressive intermittent fasting from 12 h fast to 83 h fast from March 15th to June 15th, 2024.
The participant was meeting the current physical activity guidelines in all three phases, almost exclusively through walking outside (i.e., ≥150 mi of MVPA per week; Table 2). The patient’s average resting HR went from 72 ± 2 bpm in phase 1 to 64 ± 4 bpm in phase 2 to 59 ± 3 bpm in phase 3. HRV was similarly improved (20.6 ± 2.7 ms at phase 1, 22.6 ± 4.3 ms, and 29.5 ± 5.3 ms and phase 2 and 3 respectively; Table 2).
Barriers, Facilitators, and Adverse EventsThe participant highlighted CGM use as the key factor in achieving remission using IF, enabling her to self-manage and remain confident that she was not having significant or prolonged hypoglycemia. CGM cost and lack of associated health insurance coverage were barriers that needed to be overcome. She emphasized the support from her family in attaining her goals was crucial to success. Online fasting support groups, podcasts, blog posts, and books about fasting were also helpful in learning and encouraging success. The patient also noted a more pronounce hunger sensation during the luteal phase of her menstrual cycle, making fasting more difficult during this period. No serious adverse events were noticed during her fasting regime, even when performing prolonged fasting (i.e., 83 h fast; more information provided in Supplementary Material). The patient mentioned that her sleep has become more restful, without needing to wake 2–3 times at night to use the bathroom, significantly enhancing both the quantity and quality of her sleep as underlined by her smart watch data (Supplementary Material Table S1).
The present case study underlines the possibility of achieving T2D remission with IF and physical activity outside supervised clinical and research environments. It also suggests that T2D remission may be possible for individuals with a diagnosis ~15 years, with high blood glucose levels, and taking insulin—factors traditionally thought to hinder the possibility of remission [5]. It could be speculated that a more aggressive dietary approach, such as fasting, may be a viable alternative for individuals further along in disease progression compared to a low-calorie or low-carbohydrate diet.
An important aspect mentioned by the patient was the utility and necessity of having a CGM during this process, especially since she was undertaking this endeavor without the assistance of a healthcare professional or physician. She noted that the CGM provided a sense of security by allowing her to see real-time variations in her blood glucose levels and make changes accordingly. It is important to emphasize that we do not recommend stopping medication without consulting a professional. The patient mentioned previous negative experiences with various physicians and felt that their lack of knowledge about non-pharmaceutical approaches hindered her ability to take control of her health. She changed family doctors because of this and now feels supported in this novel approach. This highlights the need for educating healthcare providers about non-pharmaceutical approaches (including IF) and the possibility of T2D remission, a concept that has not yet reached most of the Canadian population. Therefore, knowledge mobilization initiatives, such as www.diabetesremission.ca (Accessed 20 May 2025), are crucial for increasing awareness and providing free, credible information about T2D remission. Healthcare providers and clinicians are encouraged to consult the review by Grajower and Horne (2019), which provides an excellent overview of the mechanisms, benefits, risks, management strategies, and medication adjustments related to intermittent fasting in individuals living with T2D [21].
The results of this case showing substantial weight loss coinciding with T2D remission are in line with previous studies showing that weight loss was associated with a greater chance of achieving T2D remission. For instance, 85% of the individuals who lost more than 15 kg after 1 year achieved T2D remission in the DiRECT trial [6]. In the present case study, the patient lost a total of 13.8 kg in 97 days. One interesting finding that may require more attention is the distribution of that weight loss with more than half of the weight loss after 28 days being attributable to reduced lean body mass (-5.8 kg). Although a proportion of this reduction in lean body mass may reflect liver and muscle glycogen, and consequently water weight [22], this reduction remains noteworthy. In the absence of physical capacity or overall strength measured before and after the T2D remission status, we are unable to determine if the reduced LBM was associated with muscle strength loss. It is crucial that studies assessing remission measure change in body composition and concomitant modulation in muscle quality (i.e., muscle strength), especially in older populations where maintaining physical capacity is essential to preserve autonomy.
Finally, another interesting finding from this study was the reduction in resting HR as well as the improvement in HRV. In the National FINRISK Study (n = 28,047) resting HR showed a strong graded relationship with the incident cardiovascular disease even when controlling for traditional cardiovascular risk factors (e.g., age, total cholesterol, physical activity, systolic blood pressure, body mass index, etc.), [23]. Therefore, the reduction in resting heart rate of ~12 bpm is a potentially clinically relevant reduction that could reduce the overall cardiovascular stress, ultimately reducing CVD risk.
The results of the present investigation should be interpreted in light of its limitations. Considering the nature of the case study, it is difficult to generalize the findings, which reinforces the need for randomized controlled trials to investigate the effect of IF on T2D remission, particularly in participants with more advanced stages of type 2 diabetes. To limit potential researcher’s bias, the full article was revised by the patient assuring accurate representation of their story and experience. All data was collected by the patient on their own accord with no request from the researchers or initial plans to publish a case study; this is a strength as it reduced any unintended influence on behavior that may have modulated the results. Additionally, given the nature of this case study, we were unable to report changes in other health parameters such as blood pressure and lipid profile, which would have provided a more comprehensive picture of the potential effects of intermittent and short-term fasting. However, the fact that the participant was using CGM and her Apple Watch continuously for more than 4 months and performed multiple DXA scans were strong points of the present study. Finally, it is important to acknowledge that IF may not be suitable for all individuals. Those with underlying conditions such as eating disorders or taking certain medications may experience adverse effects if fasting is undertaken without medical supervision. As with other therapeutic strategies, (e.g., exercise and pharmacological interventions), healthcare providers should tailor fasting protocols based on each patient’s health status, needs and preferences.
This case study underlines the possibility of achieving T2D remission with IF and physical activity in real-world settings outside clinical and research environments which may help generate hypotheses or inform future research confirm those findings. We highly recommend clinicians and healthcare providers to deepen their understanding of T2D remission and the potential adoption of non-pharmacological approaches such as IF and physical activity to help support patients wanting to reduce medication use and self-management their condition.
The following supplementary materials are available online: https://doi.org/10.20900/agmr20250009, Supplementary Figure S1: Week by week fasting regiment during phase 3; Supplementary Figure S2: Average glucose profile during the patient’s 83 h fast; Supplementary Table S1: Sleep quantity and quality during phase 1, 2 and 3.
The University of British Columbia Ethics Board has been consulted regarding the case report. According to Guidance Note 4.4.2 on Case Reports, individual case reports do not require ethical review as long as the patient is informed that a report may be published, and written consent is obtained, which has been done in this case.
The datasets from the current study are available from the corresponding authors upon reasonable request.
Conceptualization, AMC, HI, and JPL; Methodology, AMC, HI, and JPL; Validation, AMC, APG, HI, and JPL; Formal Analysis, AMC, HI, and JPL; Investigation, APG; Resources, JPL and HI; Data Curation, AMC, APG, HI, and JPL; Writing—Original Draft Preparation, AMC; Writing—Review & Editing, AMC, APG, HI, and JPL; Visualization, AMC, APG, HI, and JPL; Supervision, HI, JPL; Project Administration, HI, JLP.
JPL is volunteer Chief Scientific Officer for the Canadian registered charity, the Institute for Personalized Therapeutic Nutrition and holds founder shares in Metabolic Insights Inc., a company that developed non-invasive metabolic monitoring devices. The authors declare there are no competing interests.
JPL is supported by a UBC Okanagan Principal’s Research Chair in Metabolism. AMC was supported by the CIHR, FRQS and Michael Smith Research BC postdoctoral fellowship, HI was supported Michael-Smith Health Research Trainee Award.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
Marcotte-Chénard A, Pinsonneault-Grenier A, Islam H, Little JP. Achieving Type 2 Diabetes Remission in Real-World Settings with Intermittent Fasting: A Case Study. Adv Geriatr Med Res. 2025;7(2):e250009 https://doi.org/10.20900/agmr20250009

Copyright © Hapres Co., Ltd. Privacy Policy | Terms and Conditions